Na2 2H2O _ 2NaOH H2 e. N2 g 3H2 g - 2NH3 g How many liters of hydrogen gas are required to product 2 moles of NH3 g.

Balancing Chemical Equations Ppt Download
B Gaseous butane C 4 H 10 reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor.

. A A2 is the limiting reactant. H2O l energy -- H2O g 2. The Law of Conservation of Mass states.
Which one of the following is a balanced chemical equation. 2 question Which of the following choices represents a balanced chemical equation. The following balanced chemical equation represents the burning of octane one of the components of gasoline used to fuel engines.
That matter can only be changed into new substances by introducing a catalyst. What is the balanced chemical equation for this reaction and what is the limiting reactant. Which of the following choices represents a balanced chemical equation.
2Na 2H2O _ 2NaOH H2. 1 Answer anor277 Jul 21 2018 Ones that represent the formation of new substances and the making and breaking of strong chemical bonds. The following diagram represents the reaction of A2 unshaded spheres with B shaded spheres.
CH 4 O 2 CO 2 H 2 O. H2O l --H2O s energy. Same number of each kind of atom appears in the reactants and in the products.
2A_2 B rightarrow A_4B. Which of the following represents a balanced equation for combining sodium metal Na with water H20 to produce NaOH and hydrogen gas H2. H2SO4 Ba OH2 BaSO4 H2O.
A_2 is the limiting reactant. Na H2O _ NaOH H2 b. Which of the following balanced equations represents a chemical change.
Use uppercase for the first character in the element and lowercase for the second character. A chemical reaction in which two or more substances undergo a chemical union to form a more complex substance is a _ reaction Weegy. The measure of the number of atoms in one element that will combine with an atom of another element is ValenceUser.
Fe Au Co Br C O N F. 2Ca3 PO42 6SiO2 P4O10 6CaSiO3. This unbalanced equation represents a chemical reactionPbNO32 NaI NaNO3 PbI2What is the coefficient for NaI once the equation is balanced.
That matter exists in all states and reacts the same. That matter exists in the same state throughout any chemical change. The balanced equation will appear above.
A2 4 B2 2 AB4. Which of the following represents an incorrectly balanced chemical equation. 4AL s 3O2 g - 2AL2O3 s Hydrogen gas and nitrogen gas can react to form ammonia according to the following equation.
To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. A2 is the limiting reactant. 2na 2h2o 2naoh h2 Write the balanced chemical equation for the reaction shown.
Subscripts of the reactants equal the subscripts of the products. Na H3O _ NaOH H2 c. 2 KHCO3 K2CO3 H2O c.
Coefficients of the reactants equal the coefficients of the products. CO 2 2H 2 O 2CH 4 2O 2. CaPOa2 6SIO - Pa010 3CaSio3 O B.
2H2O l energy --2H2 g O2 g 4. And such chemical reactions CONSERVE both mass and charge. A Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas.
Chemistry questions and answers. What balanced equation represents a chemical change. Products and reactants are the same chemicals.
Write a balanced molecular equation describing each of the following chemical reactions. Ca3po42 6sio2 p4o10 3casio3 2ca3po42 6sio2 p4o10 Subjects English. B A2 is the limiting reactant.
2C8H18g 25O2g -- 16CO2g 18H2Ol How many molecules of carbon dioxide are represented by the equation. Which of the following correctly represents a balanced chemical equationA Fes 4H2Og Fe3O4s 4H2gB 3Fes 4H2Og Fe3O4s 4H2gC 3Fes H2Og Fe3O4s H2gD 3Fes 4H2Og Fe3O4s H2gAnswer -A balanced chemical equation is one in which the number of atom. Ca3 PO42 6SiO2 P4O10 3CaSiO3.
A 4 B AB4. A chemical reaction in which two or more substances undergo a chemical union to form a more. 4A_2 6B rightarrow 2A_4B B is the limiting reactant.
A chemical equation is balanced when the a. B is the limiting reactant. NH4NO3 N2O 2 H2O d.
2CaPOa2 3SIO- 2P4010 t2Casioz OD. H2 O2 H2O b. D 4A2 6B 2A4B.
Which of the following is the balanced chemical equation that represents the chemical reaction of potassium. Na H2O _ NaOH H d. Identify the limiting reactant and a balanced equation for the reaction.
H2O g -- H2O l energy. 108 The following diagrams represent the reaction of A2 shaded spheres with B2 unshaded spheres. Correct answer - Which of the following choices represents a balanced chemical equation.
Which of the following is a balanced chemical equation for the synthesis of nabr from na and br2 Is the following chemical equation balanced. 2Ca3 PO42 3SiO2 2P4O10 2CaSiO3.
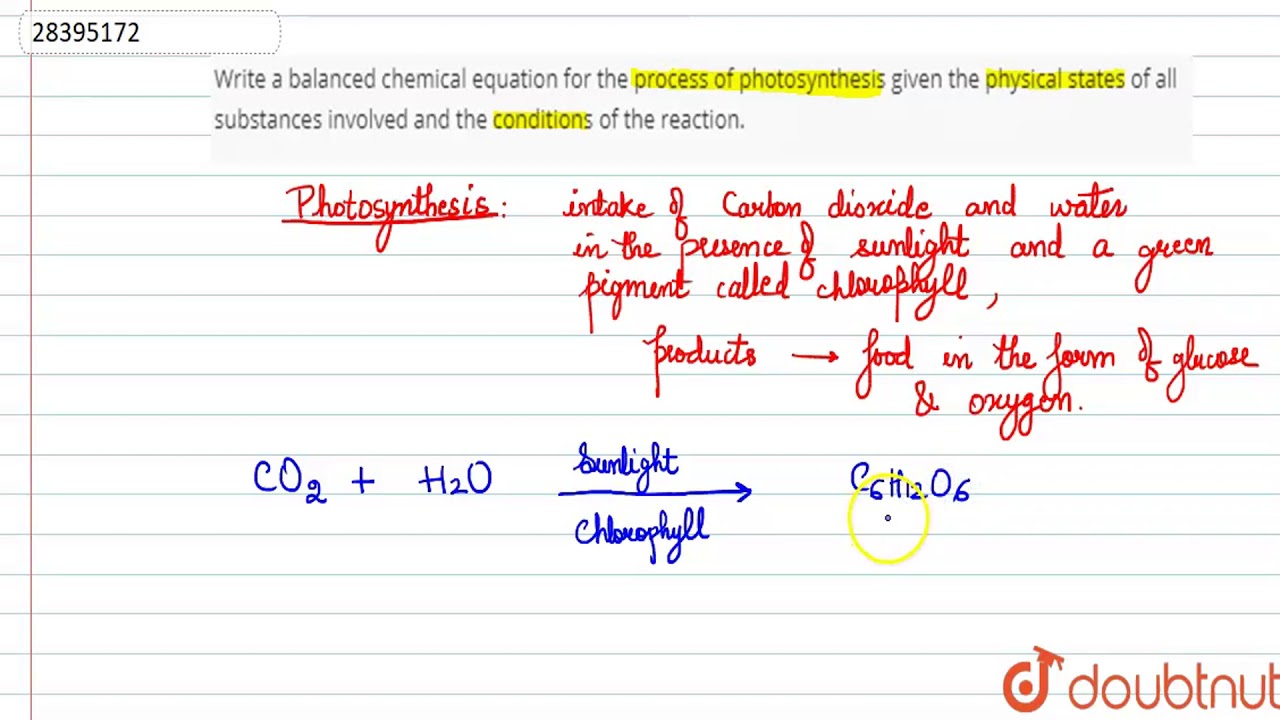
Write A Balanced Chemical Equation For The Process Of Photosynthesis Given The Physical States Of Youtube
Chem 101 General Chemistry Topic

Practical Centre Introduction To Chemical Kinetics Mcqs Chemistry Xi Chemical Kinetics Chemistry Chemistry Basics

Balancing Chemical Equations Ppt Video Online Download

Balancing Chemical Equations Ppt Download

7 2 The Chemical Equation Balancing Chemical Equations Introductory Chemistry

Answers 2 C 13 C 3 B 14 D 4 C 15 D 5 B 16 D 6 A 17 A 7 C 18 D 8 A 19 B 9 D 20 D 10 A 21 A 11 B 22 B 12 C Ppt Download


0 Comments